
Spring 2021
Making Responsible Choices in Digital Health Research
Camille Nebeker, EdD, MS✉ and Rebecca J. Bartlett Ellis, PhD✉; Behavioral Informatics and Technology SIG
Looking for that perfect wearable technology or mobile app for your research study? With so many technologies available, how do you know which ones to choose? Cost is certainly something to consider. Beyond cost, what other factors come into play when selecting a technology for your digital health research?
Digital health research findings show great promise for improving health and are beginning to directly influence health policies and practices so that millions of people can lead healthier lives. However, to do digital health research ethically and responsibly requires that we, the researchers, think prospectively about possible risks of harm and then, work to fix rather than break things2–4. To help guide your decision-making process, we introduce the Digital Health Checklist (DHC), which is organized across an interconnected framework below1.
We initiated research that resulted in the Digital Health Checklist and Framework (see Figure 1) to address a gap in available guidance. The DHC was developed with a group of subject matter experts and further evaluated by digital health researchers affiliated with the Society of Behavioral Medicine1. The resulting framework is grounded by ethical principles and consists of four intersecting domains identified as critical to decision-making including: 1- access and usability, 2- risks and benefits, 3- privacy, and 4- data management.
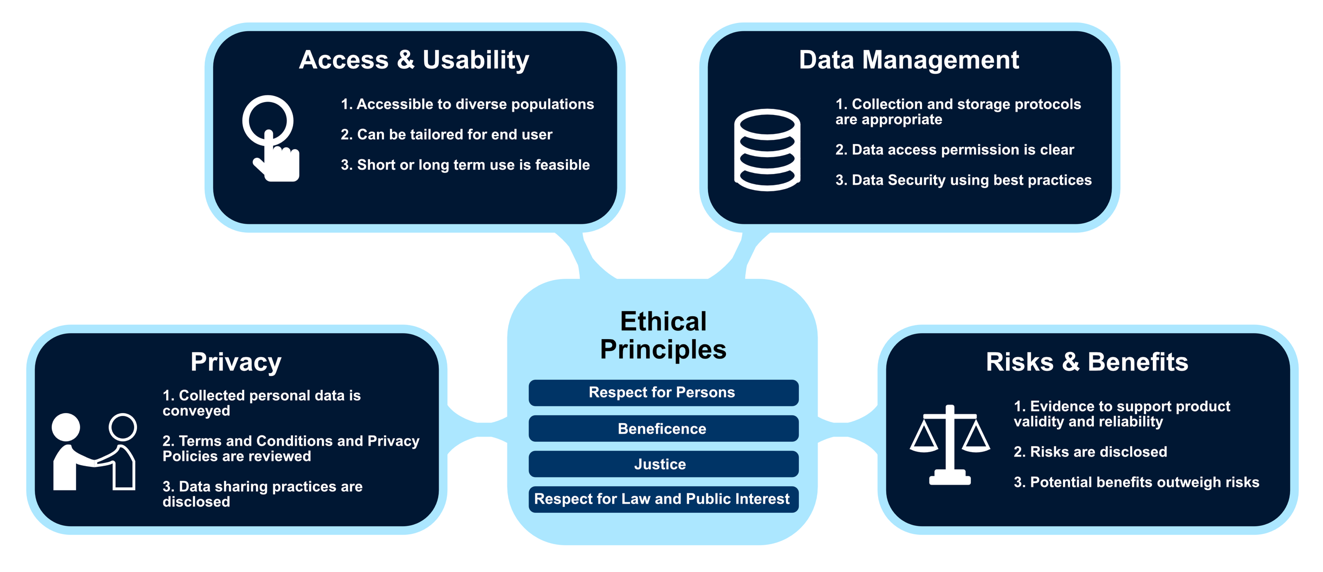
Figure 1: Digital Health Framework with examples of checklist prompts embedded within each domain. (Used with permission of C. Nebeker, ReCODE Health)
The DHC-R is licensed under a Creative Commons Attribution-Non-Commercial 4.0 International License (2018-2020) and available at https://recode.health/tools/.
Digital Health Checklist Domains
Privacy prompts researchers to consider the types of personal information that is collected about participants. For example, commercial digital tools include Terms of Service and Privacy agreements that should be read by researchers. These agreements may collect, store, and share personal information that conflict with participant expectations.
Access and Usability focuses on whether the participant can access and use the proposed device. For example, consider whether the participant will need a certain version or type of device, whether Internet or Bluetooth access is needed and whether costs might be associated with these requirements. Whether the product/tool has been tested with the target or similar populations is also important to consider.
Data Management is a broad topic and includes how data collected, stored, shared and the extent to which the data are accessible with other systems or interoperability. Considering how the research team will manage participant data as it is collected by digital tools, how data will be transmitted and the interface with other digital products influences risk assessment. Lastly, whether and how data will be shared with research participants is increasingly important to factor in prospectively.
Risks and Benefits involves an evaluation of the probability and magnitude of potential harms and discomforts weighted against the potential benefits of knowledge to be gained from the study. Risks assessment includes defining the type of harm (e.g., physical, economic, psychological) along with duration, severity, and intensity. Risk assessment is increasingly nuanced and complex in the digital age.
For more information, contact Drs. Nebeker and Ellis or visit the ReCODE Health resources at https://recode.health. ReCODE Health is a global center physically located at UC San Diego. Our team and collaborators conduct research and also provide education and consultation on aspects of digital health. You can access our ReCODE Health publications to learn about our research and, if you want to use the DHC, check out our beta version of the checklist online and share your feedback.